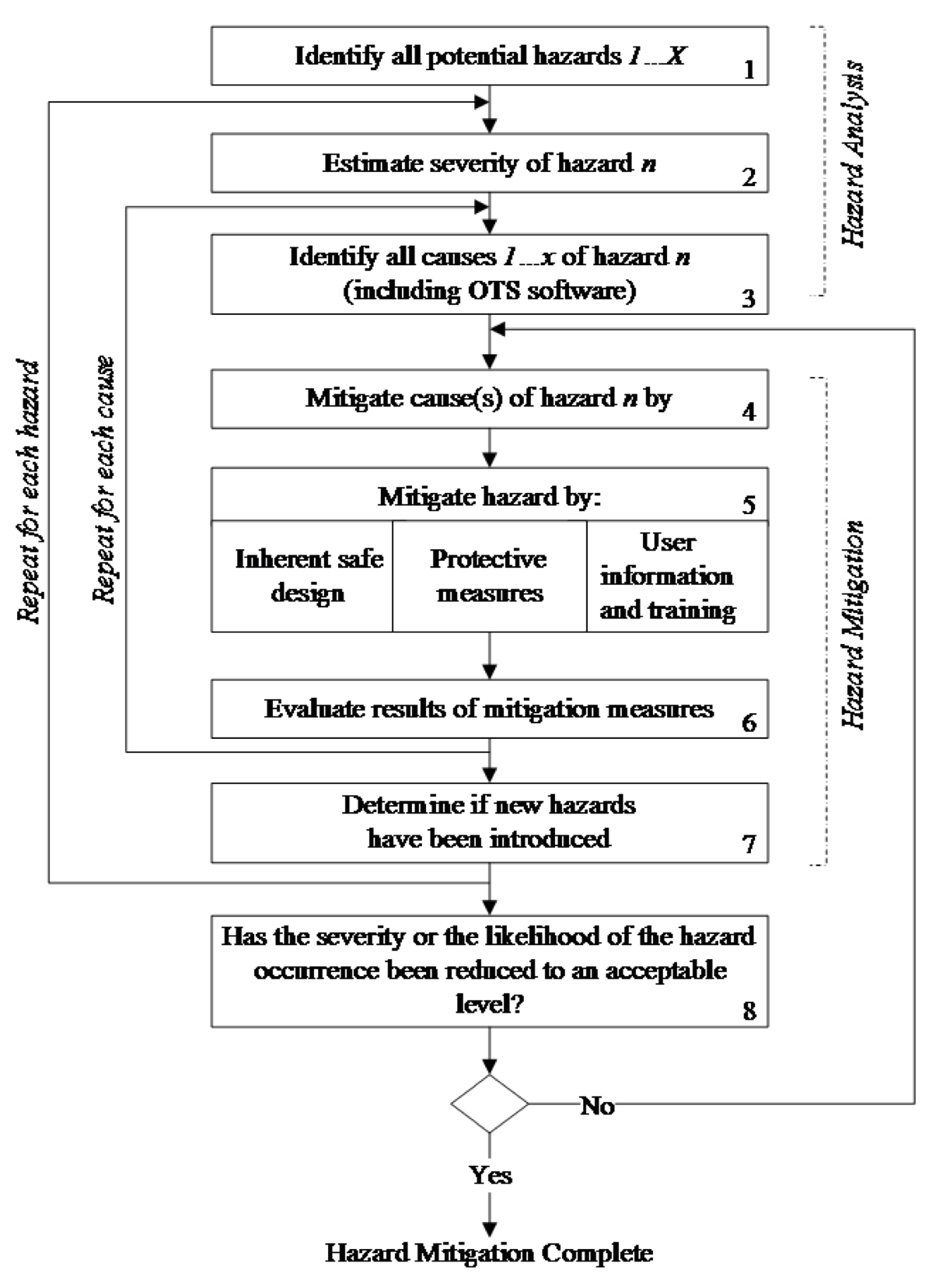
Off The Shelf Software Fda . this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket.
from innolitics.com
this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket.
2019 FDA Guidance OffTheShelf Software Use in Medical Devices
Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors.
From itenterprise.co.uk
The Complete Advantages and Disadvantages of Off the Shelf Software Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From dcastalia.com
Custom Software for A Better Choice Over Offshelf Software Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From www.regdesk.co
FDA Revised Guidance on OfftheShelf Software Applications RegDesk Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From diceus.com
Custom vs OfftheShelf Software Advantages & Disadvantages Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From blog.foreworth.com
The Lowdown on OfftheShelf Software Advantages and Disadvantages Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From radixweb.com
Difference between Bespoke Application Development and OfftheShelf Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From www.slideserve.com
PPT Hardware and Software PowerPoint Presentation, free download ID Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From www.scribd.com
Guidance OffTheShelf Software Use PDF Medical Device Risk Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From issuu.com
Difference Between Proprietary Software and OffTheShelf Software by Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From www.cleverdevsoftware.com
Custom Software Vs. Off The Shelf The Pros & The Cons CleverDev Software Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From www.botreetechnologies.com
Customized Software What is it, Types, and Examples Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From www.web-alliance.co.uk
Pros and cons of offtheshelf software. Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Fda this guidance document provides information regarding the recommended documentation sponsors. to ensure device manufacturers provide the appropriate documentation for any premarket. Off The Shelf Software Fda.
From xbsoftware.com
Custom Software vs OfftheShelf Software XB Software Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Introduction RegDesk Off The Shelf Software Fda to ensure device manufacturers provide the appropriate documentation for any premarket. this guidance document provides information regarding the recommended documentation sponsors. Off The Shelf Software Fda.